Shaping Future Cancer Care: Analyzing Immuno-Oncology Trends in Top Pharma and Beyond
Introduction
The field of oncology continues to experience significant expansion, particularly in the realm of Immuno-Oncology (IO) drugs. A vast clinical development pipeline aims to transform the existing cancer treatment paradigm. The Sedulo Group team rigorously analyzed pharma-sponsored clinical trials initiated between 2018 and 2022 for unapproved primary IO drugs and uncovered key trends and innovative therapeutic strategies across Top 20 Pharma and All Other Pharma. Read our blog Know where your competitors are conducting clinical trials to review part one of this series.
Approach
In order to understand how the Top 20 Pharma companies’ R&D efforts compare to other pharma companies, Sedulo used the Scrips 100 Rankings to categorize clinical trials by Top 20 Pharma and All Other Pharma. This method pinpoints the R&D priorities of these two groups across different drug classes.
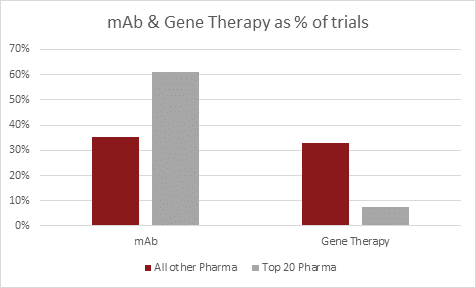
The bar graph below presents the distribution of various drug types across the two groups.

Note:
The Monoclonal antibody (mAb) umbrella group encompasses human, humanized, murine, chimeric, and other subgroups.
The Gene Therapy umbrella group encompasses CAR, TCR, TILs, and other subgroups.
What are the leading Therapeutic Classes by %?

Notes:
Abbreviations: BiTE = Bispecific T-cell Engager; BsAb = bispecific antibody; CAR = Chimeric Antigen Receptor, mAb = monoclonal antibody, TCR = T-cell Receptor,
Sources:
- Trialtrove ǀ Citeline, February 2023
- Scrip 100 Top 20 sponsors were updated in January 2023 per PharmaProjects. Rankings are based on fiscal 2021 drug sales
Key Takeaways from the Assessment
Sedulo Group’s analysis of trial clusters, registries, and various IO drug types reveals several critical insights:
- Monoclonal antibodies (mAbs) remain the primary drug type for both the Top 20 Pharma and All Other Pharma groups. However, these groups show distinct preferences in mAbs subgroups. The Top 20 Pharma predominantly focus on humanized mAbs in their clinical trials, while All Other Pharma mainly concentrate on human mAb types.
- Gene therapy emerged as the 2nd largest umbrella group evaluated by All Other Pharma. Within this category, Chimeric Antigen Receptor (CAR) therapies are the most extensively studied subgroup.
- Bispecific T-cell Engagers (BiTEs) emerged as the 2nd largest group under clinical investigations by the Top 20 Pharma.
Where does this lead us?
Despite the rise of multiple novel classes of therapeutics in cancer, mAbs, BiTEs, and CARs continued to dominate the clinical trials initiated between 2018-2022. This raises several important questions about the evolving clinical landscape:
- Will mAbs remain the cornerstone of cancer therapeutics?
- The future of mAbs hinges on the success of novel antibody-based therapies in achieving maximum specificity with minimal toxicity. The clinical profile of mAbs will be influenced by multiple factors, including immunogenicity and their domain origin, whether human, humanized, murine, or chimeric.
- Will BiTEs revolutionize the cancer treatment landscape and become the preferred therapeutic approach?
- Assessing this potential requires a better understanding of the risk/benefit profile of bispecifics compared to cell-based therapies and other novel drug types.
- Can next-generation CAR, allogenic CAR, or CAR-NK therapies overcome the hurdles of safety and manufacturing complexities?
- If they do, how will small- and mid-size biotech/pharmaceutical companies effectively manage the intricate commercial aspects associated with CAR-T therapies, which demand considerable expertise?
- How will these novel drug types co-exist or complement each other in the future cancer treatment landscape?
- Continual monitoring of the developing cancer treatment landscape is critical to understanding the future SOC.
Competitive Intelligence and Strategic Insights Drive Success
Oncology is a fiercely competitive field, with numerous novel therapies in different stages of discovery, development, and commercialization. This dynamic ecosystem is driven by catalysts like new drug discoveries, trial initiation, data readouts, regulatory processes, approvals, and product launches.
Navigating this dynamic landscape successfully requires a holistic view of the competitive environment. Strategic insights and fact-based data are vital to identify threats and opportunities and to formulate short- and long-term business strategies.
Sedulo Group is uniquely equipped to guide you through this evolving treatment paradigm. Our expert team utilizes a proprietary approach to research and insight development, employing a systematic “Four I’s” process. This involves gathering information, analyzing intelligence, uncovering insights, and contextualizing implications. This rigorous methodology ensures that clients gain a holistic view of the competitive landscape, empowering them to anticipate and understand future competitor and market developments.
Are you searching for a strategic partner to assist your team in successfully navigating this complex and dynamic environment?
Schedule your free consultation and discover how Sedulo Group can support your business!